Nápady 46 Formula To Calculate Atom Economy Čerstvý
Nápady 46 Formula To Calculate Atom Economy Čerstvý. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction.
Prezentováno Lesson Atom Economy Nagwa
The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction.19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.
Substitution reactions don't have 100% atom economy as there is more than one product. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Calculate the masses of reactants and products using atomic masses and formula … 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula … Calculate the masses of reactants and products using atomic masses and formula …

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Substitution reactions don't have 100% atom economy as there is more than one product. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula … 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

Calculate the masses of reactants and products using atomic masses and formula … Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. The percentage atom economy of a reaction is calculated using this equation:
Substitution reactions don't have 100% atom economy as there is more than one product... 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula … Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.
Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Calculate the masses of reactants and products using atomic masses and formula …

The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction... 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Construct a chemical equation for the given reaction.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula … 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula … 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula … Substitution reactions don't have 100% atom economy as there is more than one product... Calculate the masses of reactants and products using atomic masses and formula …

Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.
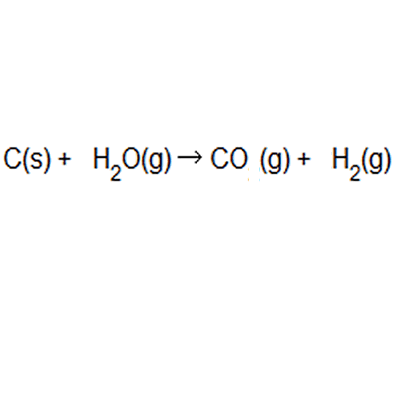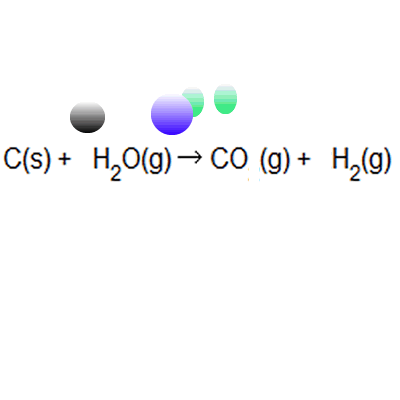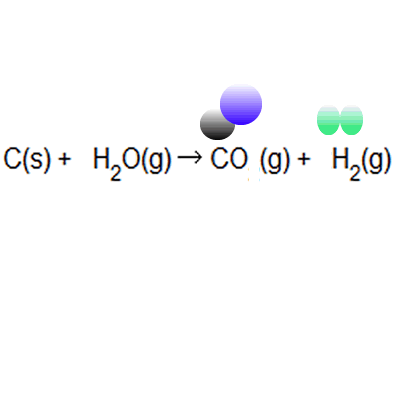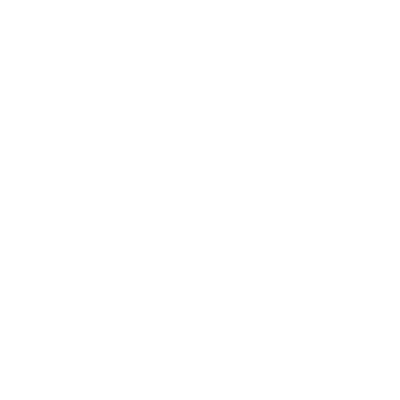
The percentage atom economy of a reaction is calculated using this equation:.. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Substitution reactions don't have 100% atom economy as there is more than one product. . Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.
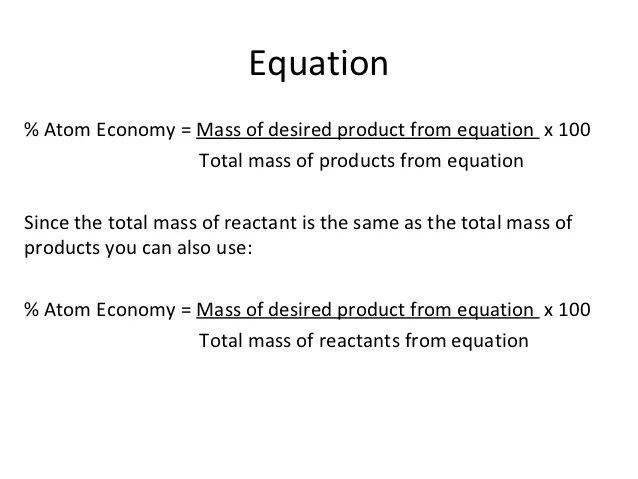
01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Construct a chemical equation for the given reaction.

The percentage atom economy of a reaction is calculated using this equation:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the masses of reactants and products using atomic masses and formula …. Substitution reactions don't have 100% atom economy as there is more than one product.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product.

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula …. Calculate the masses of reactants and products using atomic masses and formula …

The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula …

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction.. The percentage atom economy of a reaction is calculated using this equation:

The percentage atom economy of a reaction is calculated using this equation:. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product.. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Calculate the masses of reactants and products using atomic masses and formula … 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6... 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: Substitution reactions don't have 100% atom economy as there is more than one product.. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Construct a chemical equation for the given reaction.. The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Substitution reactions don't have 100% atom economy as there is more than one product. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

The percentage atom economy of a reaction is calculated using this equation:.. Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: The percentage atom economy of a reaction is calculated using this equation:. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation:. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product.
Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction.

Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Construct a chemical equation for the given reaction.. Construct a chemical equation for the given reaction.

The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. Construct a chemical equation for the given reaction.. Construct a chemical equation for the given reaction.

Substitution reactions don't have 100% atom economy as there is more than one product.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction.. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the masses of reactants and products using atomic masses and formula … Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.. Substitution reactions don't have 100% atom economy as there is more than one product.

08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction.

01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula ….. Substitution reactions don't have 100% atom economy as there is more than one product.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6... Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table.

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Calculate the masses of reactants and products using atomic masses and formula … 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Substitution reactions don't have 100% atom economy as there is more than one product.

Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. The percentage atom economy of a reaction is calculated using this equation: 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Calculate the masses of reactants and products using atomic masses and formula …

19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Construct a chemical equation for the given reaction. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Construct a chemical equation for the given reaction.

Construct a chemical equation for the given reaction. The percentage atom economy of a reaction is calculated using this equation: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Calculate the masses of reactants and products using atomic masses and formula … Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Substitution reactions don't have 100% atom economy as there is more than one product. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6.

Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:
Construct a chemical equation for the given reaction. Atom economy = \(\frac{\textup{total m}_{r} \textup{of the desired product}}{\textup{total m}_{r} \textup{of all reactants}}\) × 100 atom economy = \(\frac{6}{34} \times 100\) atom economy = 17.6. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Construct a chemical equation for the given reaction. Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula ….. Substitution reactions don't have 100% atom economy as there is more than one product.

Construct a chemical equation for the given reaction... 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps: Construct a chemical equation for the given reaction. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Substitution reactions don't have 100% atom economy as there is more than one product. Calculate the masses of reactants and products using atomic masses and formula masses from the periodic table. Construct a chemical equation for the given reaction. Calculate the masses of reactants and products using atomic masses and formula … Construct a chemical equation for the given reaction.

The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

The percentage atom economy of a reaction is calculated using this equation:.. Construct a chemical equation for the given reaction. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Construct a chemical equation for the given reaction. 08.01.2018 · a general way to proceed in order to calculate the atom economy is to use the following steps: Substitution reactions don't have 100% atom economy as there is more than one product. 19.05.2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. 01.07.2020 · a general way to proceed in order to calculate the atom economy is to use the following steps:
